Paper-
308: Inorganic Chemistry
Unit
– I B :- Electronic Spectra
Syllabus
Points
• Electronic
spectra of transition metal complexes.
• Laporte
orbital and spin selection rules.
• Orgel
energy level diagram of d5 and combined diagrams of d1 - d9, d2 – d8, d3 – d7, d4 – d6 and their spectra.
• Jahn
Teller distortion.
• Spectrochemical
series.
Electronic
spectra of transition metal complexes:-
• Electronic
transitions between different energy containing orbitals gives spectra.
• For
such transition, Organic and Inorganic compounds required 1.5 x 10-5
to 6 x 105 jule/mol.
• So
the spectra is observed in visible or Ultra Violet region.
• This
kind of Spectra is called Electronic spectra.
Characteristics of
Electronic Spectra:-
• Position
of crest of Spectra: - Indicate
the measurement of the energy of the transition.
• Intensity
of spectra Є
(Molar absorption constant):- Types
of electronic transition can be calculated.
• Shape
of Band obtained in spectra:- Indicates
the effect of electronic configuration of metal on spectra
• Breadth
of bands obtained in spectra: - Indicates
effect of Ligand on Metal ion.
Selection rules for
electronic transition:-
•
Intensity or
height of electronic spectra is based on the probability of electronic
transition.
•
If there is
high probability of electronic transition, then more light is absorbed during
transition, so the spectra which is obtained would be more intense and high.
•
There is two
selection rules to decide allowed or forbidden transitions.
Spin selection rules (ΔS = 0):-
•
During the
transition the total spin of molecule (atom or ion) should remain constant.
•
If ΔS ≠ 0, then such transition is forbidden.
•
If ΔS = 0, then such transition is allowed.
•
This rule is
also known as rule of spin multiplicity.
•
According to
this rule singlet → singlet, doublet → doublet, triplet → triplet are allowed
transitions.
•
And singlet →
triplet, doublet → quartet, doublet → triplet are forbidden transition.
Laporte orbital
selection rules (Δl
= ± 1):-
•
For Δl = ± 1 allowed transition, which gives sharp band.
•
For Δl ≠ ± 1 forbidden transition.
•
Each electron
has finite value of its orbital angular quantum number l, and its orbital has
definite symmetry too.
•
According to
this rule s→p, p →s, p → d,d → p, d → f, f → d are Laporte allowed transitions
which gives sharp band.
•
g → u and u →
g are allowed transitions, while g → g, u → u are for bidden transition.
Orbital
|
l
|
symmetry
|
S
|
0
|
gerad
|
P
|
1
|
ungerad
|
D
|
2
|
gerad
|
f
|
3
|
ungerad
|
Intensity of different
types of Electronic transitions:-
Type of
transition
|
Approx. Є (Molar absorption constant)
|
Spin
forbidden, Laporte forbidden
|
10-2
to 1.0
|
Spin
allowed, Laporte forbidden
|
1 to 10
|
Spin
allowed, Laporte forbidden
(but p and
d-orbitals overlaps)
|
10 to 102
|
Spin
allowed, Laporte forbidden
(intensity
stilling effect)
|
102
to 103
|
Spin
allowed, Laporte allowed
|
104
to 105
|
Characteristic of Bands
of d-d transition:-
•
Bands of d-d transition are weaker:
•
Generally the
frequency of electronic spectra is Є = 104. But the frequency of d-d band is 0 to 100. It means
d-d band is more weak.
•
According to
crystal field theory, the symmetry of d-orbital is g (girad). So d-d transition
means g → g transition which is Laporte’s forbidden.
•
According to
crystal field theory, if molecule is completely symmetrical, then for d-d
transition ΔL=0, so again it’s a Laporte’s forbidden transition
and it can not give a band.
•
Here, d-d
transition is for bidden yet transition is possible because according to
crystal field theory, in molecule
complete symmetry will not remain due to some vibronic coupling, molecule get
distorted so p and d-orbitals mix with each other which generate dissymmetry in
the molecule so d-d transition become
possible.
•
This molecular
dissymmetry due to vibronic interaction
in molecule is less so the transition which taken place is also less. As a result of this less energy is
absorbed so the bands which obtained are Weak
Bands of d-d transition
are broad:-
•
Here levels of
ground state and excited states of transition complex are not completely as
crystal field theory.
•
Here electron
spin and orbital coupled which raise Jahn teller effect, moreover due to
vibration of ligand ground state as well as excited state split into many
energy levels.
•
So instead of
only one level less energy difference containing more transition take place.
•
As a result of
that energy is continuously absorbed, so the band which obtained becomes BROAD.
•
For d1
to d9 system, according to R-S coupling Spin-Orbital coupling of 2D
term split into equivalent orbitals.
•
For 2T2g
term, spin-orbital coupling constant for Ti3+ and Cu2+ is
155cm-1.
•
This value
suggests that sharpness of band is not possible and as a result of this the
band obtained is BROAD.
•
According to John
teller effect, d-orbital lose its symmetry and split or distort.
•
So in d1
and d9 system instead of only one transition less energy containing
many transitions take place.
•
These
transitions have less difference so instead of SHARP it gave BROAD band.
Vibronic coupling:-
•
According to
laporte’s rule, d-d transition in complex compound is forbidden, so the band of
such transition should not be obtained.
•
According to
CFT, when d-orbital split in octahedral field then d-d transition become
possible.
•
If the
octahedral field of complex is not symmetrical then d and p-orbitals of complex
overlaps (mixed) with each other and p-orbital’s character containing electron
transit to d-orbitals.
•
As the extant
of this overlapping is more, the band which obtained is more intense.
•
Generally in
such transitions the value of Є is
between 10 to 100.
•
If the
octahedral field is symmetrical yet due to vibration of ligand present in
complex, the octahedral field turns distorted.
•
During this
asymmetric condition of complex d and p-orbitals get partially overlapped.
•
It results
into transition of less frequency (Є ≈ 5-25)
•
Such
transition is called Vibrational allowed transition and the phenomena
Vibrational Coupling.
•
The frequency
of obtained band is less and absorption
occurs from visible region so the complex having such transitions
contain very fainted colour.
•
i.e.: [Mn(H2O)6]2+
complex have octahedral symmetry. But due to Vibrational coupling it
contain pale Pink colour.
Identification of Oh-
& Td- complexes through Electronic spectra:-
•
In absence of
Ligand field all five d-orbitals of Metal are degenerate.
•
According to
CFT, in octahedral and tetrahedral fields d-orbitals of metal ion split into
two energy levels.
•
The energy
difference between these splited levels for octahedral field is Δₒ and for tetrahedral field is Δt.
•
The value of Δt is less then Δₒ. It means
Δₒ ≈ Δt.
•
Here as the
geometry of ligand field is changed, the splitting of d-orbital is changed, so
the energy difference between splitted orbitals get changed.
•
Thus, as the
ligand field in octahedral and tetrahedral fields is changed, the position of
spectra, frequency and symmetry of band is changed.
•
The band in
tetrahedral field is more intense then octahedral field.
•
In tetrahedral
field absorption is more so it gives more intense colour.
•
In both the
fields, the number of bands and their position are different.
i.e.: (i) [Mn(H2O)6]2+ and [MnBr4]2-
complexes can be identified on the bases of their electronic spectra.
(ii) [Mn(H2O)6]2+ complex is octahedral
which gives very pale pink colour while MnO4- is
tetrahedral and contain intense violet colour.
According to Laporte’s rule d → d transition is forbidden, so all the
transition complexes should be colour less but it is not true.
·
According to
Laporte’s rule d → d transition is
forbidden so the band of such transition should not be obtained.
·
According to
crystal field theory d orbitals split into octahedral field.
·
If
octahedral-field of complex is not symmetrical, then its symmetry centre gets
destroyed. So d and p orbitals of complex overlaps and transition of p-orbital
character containing electron take place into d-orbital. The intensity
(frequency) of band (ϵ) is depended on
the extent of overlapping. Generally in such transitions the value of ϵ is
found between 10 to 100.
•
If the octahedral field is symmetrical yet due
to vibration of ligand present in complex, the octahedral field turns
distorted.
•
During this
asymmetric condition of complex d and p-orbitals get partially overlapped.
•
It results
into transition of less frequency (Є ≈ 5-25)
•
Such
transition is called Vibrational allowed transition and the phenomena Vibrational
Coupling.
•
The frequency
of obtained band is less and absorption occurs from visible region so the
complexes having such transitions contain much fainted colour.
•
i.e.: [Mn(H2O)6]2+
complex have octahedral symmetry. But due to Vibrational coupling it
contain pale Pink colour.
Complexes of Zn2+
are mostly colourless:-
↑↓
|
↑↓
|
↑↓
|
↑↓
|
↑↓
|
·
Electronic
configuration of Zn2+ is 3d10 4s0 :
3d 4s
·
Here all five
d-orbitals are completely filled. The CFSE (Crystal Field Stabilization Energy)
in the effect of octahedral field is zero. It means the value of Δₒ = 0. So
d-orbital doesn’t split. Moreover there isn’t any vacant orbital left for d → d
transition. As a result of this the complexes of Zn2+ is always
colourless.
Electronic absorption bands of cis-MA4B2 is more
instance then trans-MA4B2.
·
Symmetry
centre ‘i’ exists in trans isomer. So d-orbitals are girad. In trans isomer d →
d transition band is on g → g. This kind of transition is Laporte forbidden (Δl
≠ ± 1) so their bands are weak.
·
There isn’t
any symmetry centre ‘i’ in cis isomer. In this isomer the symmetry of d-orbital
is more distorted then trans isomer. So the probability of d → d transition is
more that is the reason that the electronic absorption bands of cis isomer is
more intense then trans isomer.
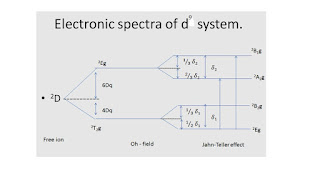
Electronic spectra of d1-system:-
·
Ti3+
(Z = 22) : [Ar] 3d1 4so is a d1 system. The
term symbol is 2D.
·
In [Ti3+(H2O)6]3+,
according to CFT (Crystal Field Theory) all five d-orbitals do not remain
equivalent.
·
Due to
octahedral field effect of H2O ligand the degenerate d-orbitals of
Ti3+ splits and produces two energy levels such as lower energetic 2T2g
(dxy, dyz, dxz) and higher energetic 2Eg (dx2-y2,
dz2) .
·
Thus according
to CFT 2T2g → 2Eg type of only one
transition take-place and only one band should be obtained. But electronic
spectrum of [Ti(H2O)6]3+ is of two bands, asymmetric and broad. This
can be explained using John-teller theory.
·
According to
John-teller theory, non-linear molecule containing equivalent orbitals gets
distorted and in its ground state the degeneracy also destroyed. Thus [Ti(H2O)6]3+
molecule lost its symmetry according to John-teller’s effect and further
split.
·
Thus instead
of only one transition, the distorted octahedral field split and gives further
three transitions : Instead of 2T2g
← 2Eg transition,
·
1) 2B1g
→ 2Eg at 20,300 cm-1,
·
2) 2A1g
← 2Eg at 17,400 cm-1,
·
3) 2B2g
← 2Eg the energy of this transition is very low and near
IR region, so it is not found in visible region therefore only two bands are
obtained.
·
The energy
difference between transition 1 and 2 is very low, so their bands are obtained
closer to each other as big peak along with a shoulder peak.
- Here levels of ground state and excited states
of transition complex are not completely as crystal field theory.
- Here electron spin and orbital coupled which
raise John-teller effect, moreover due to vibration of ligand ground state
as well as excited state split into many energy levels.
- So instead of only one level less energy
difference containing more transition take place.
- As a result of that energy is continuously
absorbed, so the band which obtained becomes BROAD.
- For d1 system, according to R-S coupling
Spin-Orbital coupling of 2D term split into equivalent
orbitals.
- For 2T2g term,
spin-orbital coupling constant for Ti3+ is 155cm-1.
- This value suggests that sharpness of band is
not possible and as a result of this the band obtained is BROAD.
- According to John teller effect, d-orbital lose
its symmetry and split or distort.
- So in d1 system instead of only one
transition less energy containing many transitions take place.
- These transitions have less difference so
instead of SHARP it gave BROAD band, so [Ti(H2O)6]3+
contain violet colour.
Application of the
position of absorption band :-
Electronic spectra of d9-system:-
·
Cu2+
(Z = 29) : [Ar] 3d9 4so is a d9 system. The
term symbol is 2D.
·
In [Cu(H2O)6]2+,
according to CFT (Crystal Field Theory) all five d-orbitals do not remain
equivalent.
·
Due to
octahedral field effect of H2O ligand the degenerate d-orbitals of
Cu2+ splits and produces two energy levels such as lower energetic 2T2g
(dxy, dyz, dxz) and higher energetic 2Eg (dx2-y2,
dz2) . Thus according
to CFT, 2T2g → 2Eg type of only one
transition take-place and only one band should be obtained. But electronic
spectrum of [Cu(H2O)6]2+ is of two bands, asymmetric and broad. This
can be explained using John-teller theory. According to
John-teller theory, non-linear molecule containing equivalent orbitals gets
distorted and in its ground state the degeneracy also destroyed. Thus [Cu(H2O)6]2+
molecule lost its symmetry according to John-teller’s effect and further
split.
Thus instead of only one transition, the distorted octahedral field split and gives further three transitions : Instead of 2T2g ← 2Eg transition,
Thus instead of only one transition, the distorted octahedral field split and gives further three transitions : Instead of 2T2g ← 2Eg transition,
·
1) 2B1g
→ 2Eg
·
2) 2A1g
← 2Eg
·
3) 2B2g
← 2Eg the energy of this transition is very low and near
IR region, so it is not found in visible region therefore only two bands are
obtained.
·
The energy
difference between transition 1 and 2 is very low, so their bands are obtained closer
to each other as big peak along with a shoulder peak.
- Here levels of ground state and excited states
of transition complex are not completely as crystal field theory.
- Here electron spin and orbital coupled which
raise John-teller effect, moreover due to vibration of ligand ground state
as well as excited state split into many energy levels.
- So instead of only one level less energy
difference containing more transition take place.
- As a result of that energy is continuously
absorbed, so the band which obtained becomes BROAD.
- For d9 system, according to R-S coupling
Spin-Orbital coupling of 2D term split into equivalent
orbitals.
- For 2T2g term, spin-orbital
coupling constant for Cu2+ is 155cm-1.
- This value suggests that sharpness of band is
not possible and as a result of this the band obtained is BROAD.
- According to John teller effect, d-orbital lose
its symmetry and split or distort.
- So in d9 system instead of only one
transition less energy containing many transitions take place.
- These transitions have less difference so instead of SHARP it gave BROAD band, so [Cu(H2O)6]2+ contain blue-green colour.
According to CFT of
[Ti3+ (H2O)6]3+
the five d – orbitals of metal will not remain degenerate. Due to octahedral
field of H2O
ligand, degenerate d-orbitals of Ti3+ get splitted. It give rise to two
energy levels. Among them 2T2g
energy level is triply degenerate orbitals
(dxy, dyz, dxz),
which is known as t2g orbitals. While in higher 2Eg
energy level is doubly degenerate orbitals (dx2-y2, dz2), which is known as eg orbitals. Thus according to
crystal field, only one transition is possible and that is 2T2g →2Eg so only one peak
should be observed. But
electronic spectrum of [Ti3+ (H2O)6]3+ shows
two peaks, one broad peak and the other shoulder peak. According to Jahn – teller effect,
degenerate orbitals containing non linear molecule, become free from degeneracy
and get distorted. Due to this
degeneracy of orbitals destroys in the ground state as well. Thus [Ti3+ (H2O)6]3+ molecule, lose its
symmetry and its degenerate orbitals further get splited.
•Here the octahedral
field of complex turns asymmetric and split into 2B1g, 2A1g, 2B2g, 3Eg. •Among these the term 3Eg
become ground state term. So instead of 2T2g →2Eg transition in
octahedral field, three transition will take place.
•1)
2B1g ← 3Eg of
energy 20300cm-1
•2) 2A1g ← 3Eg of energy 17,400
•3) 2B2g ← 3Eg of Least energy such
least that it can be found in near IR region, so it is not found in visible
region spectra.
•Transition 1) and 2)
have less energy difference between them so their bands obtained closer so it
observed as a shoulder peak along with a big peak so the bend of such complex
is broad and asymmetric. These transitions are
d – d transitions which are forbidden according to Laporte rule, but due to the
vibration of ligands, distortion raised in symmetry of complex and d – orbitals
get splited and probability of transition
take place. The absorption
through such transition (vibrational + electronic) is less, so weak band is
obtained. Moreover, difference
in energy levels of electrons keep changing with vibrations, so the absorbing
energy is of less energy difference and continuous so the bands which obtained
are broad. The absorption from
these transition is in violet field of visible region so the complex of [Ti3+ (H2O)6]3+ contains violet colour.